HOUSTON — New research from Houston Methodist concludes that plasma transfusions from recovered COVID-19 patients are a safe and at least somewhat effective treatment option for people still dealing with severe coronavirus symptoms. Out of 25 patients given a transfusion, 19 saw their condition improve and 11 were discharged.
This was the first U.S. convalescent plasma transfusion trial, and participating patients saw no negative nor adverse side effects after receiving the treatment. There have, however, been smaller studies conducted on blood plasma transfusions for COVID-19 patients. All of those projects came to similar conclusions.
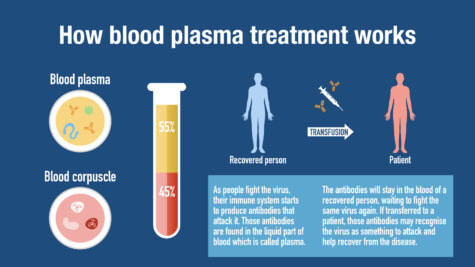
“While physician scientists around the world scrambled to test new drugs and treatments against the COVID-19 virus, convalescent serum therapy emerged as potentially one of the most promising strategies,” comments corresponding study author Dr. James M. Musser, chair of the Department of Pathology and Genomic Medicine at Houston Methodist, in a statement. “With no proven treatments or cures for COVID-19 patients, now was the time in our history to move ahead rapidly.”
This treatment approach for viral diseases certainly isn’t new; blood transfusions were used in the same way during the Spanish Flu outbreak of 1918, and more recently during the 2003 SARS pandemic, the 2009 influenza H1N1 outbreak, and the 2015 Ebola outbreak in Africa.
The first signs that plasma transfusions from recovered COVID-19 patients could help others came from China. A small number of Chinese patients showed improvement after receiving blood transfusions. Once news of that project was reported to the team at Houston Methodist, they immediately started thinking about how to apply convalescent serum therapy to COVID-19.
Many of the patients’ health outcomes following the plasma transfusions mirrored what happened to COVID-19 patents who were given the antiviral drug remdesivir. Furthermore, while some patients in this trial study did experience complications after receiving blood, the research team believe those problems were caused by the coronavirus infection itself and not the transfusions.
All of the plasma transfusions given for this trial were done on an emergency basis for patients in critical condition. With that in mind, the study’s authors say much more research is needed before the therapeutic effects of convalescent serum therapy can be determined.
A randomized control trial for just this purpose may take place at Houston Methodist in the future. Wherever it happens, that trial will focus on more specific aspects of the treatment like timing of the transfusion in reference to the emergence of coronavirus symptoms, the number and volume of transfusions (depending on the patient), and anti-body levels in the plasma. In theory, a more thorough trial will help inform doctors of the best time to administer transfusions for individual patients.
More patients have already been given plasma transfusions since this first trial. In all, 74 critically ill COVID-19 patients have received blood from recovered patients at Houston Methodist. Of that group, 50 have been discharged thus far. There’s also no shortage, it seems, of blood to work with; over 150 recovered coronavirus patients have donated so far, with many making more than one donation.
The study is published in The American Journal of Pathology.