
EVANSTON, Ill. — A revolutionary injection may be able to repair the damage from severe spinal cord injuries that leave patients without the use of their limbs. In experiments with paralyzed mice, a team from Northwestern University demonstrated that the shot helped the animals regain their ability to walk within just four weeks of a single injection.
The drug appears to be so effective that human trials could begin next year. Study authors say their breakthrough drug encourages nerves to regrow in damaged areas.
“Our research aims to find a therapy that can prevent individuals from becoming paralyzed after major trauma or disease,” says study leader Professor Samuel Stupp in a university release.
“For decades, this has remained a major challenge for scientists because our body’s central nervous system, which includes the brain and spinal cord, does not have any significant capacity to repair itself after injury or after the onset of a degenerative disease,” Stupp continues.
“We are going straight to the FDA to start the process of getting this new therapy approved for use in human patients, who currently have very few treatment options.”
Could the shot cure neurological diseases too?
In addition to mice, the technique worked on human cells grown in lab dishes. Researchers believe this injection could also help stroke victims make a full recovery. There’s a possibility it may even cure illnesses that affect the central nervous system – including Alzheimer’s, Parkinson’s, and motor neuron disease (ALS).
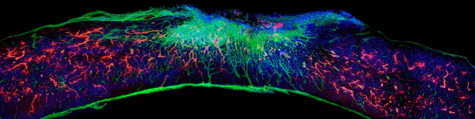
Damage to the spinal cord interrupts the constant stream of electrical signals from the brain to the body. This can lead to paralysis in any limb where signals can no longer get through.
The new medication sends bioactive signals to trigger cells to regenerate, leading to five key improvements. Long spindly parts of the severed nerves (axons) begin to regenerate and scar tissue which prevents healing disappears. Myelin, an insulating tissue, reformed around cells. In the final two improvements, functional blood vessels form to deliver nutrients to the injury site and more motor neurons survive.
The study in the journal Science finds the drug biodegrades and nourishes the cells after three months, absorbing into the body without side-effects.
Giving new hope to thousands of paralyzed patients
According to the United Spinal Association, there are nearly 300,000 Americans currently living with a spinal cord injury. About 17,900 new SCI cases occur each year. Nearly four in five of these injuries happen to men and the average age of a person who sustains such a devastating injury is around 43 years-old.
Fewer than three percent of SCI patients ever recover basic physical functions. A third return to the hospital at least once a year.
“Currently, there are no therapeutics that trigger spinal cord regeneration,” says Prof. Stupp, an expert in regenerative medicine. “I wanted to make a difference on the outcomes of spinal cord injury and to tackle this problem, given the tremendous impact it could have on the lives of patients. Also, new science to address spinal cord injury could have impact on strategies for neurodegenerative diseases and stroke.”
Researchers inject the drug as a liquid, which immediately gels into a complex network of nanofibers that mimic the extracellular matrix of the spinal cord. The cocktail of synthetic materials are then able to communicate with cells.
“Receptors in neurons and other cells constantly move around,” Prof. Stupp explains. “The key innovation in our research, which has never been done before, is to control the collective motion of more than 100,000 molecules within our nanofibers. By making the molecules move, ‘dance’ or even leap temporarily out of these structures, known as supramolecular polymers, they are able to connect more effectively with receptors.”
Increasing the ‘social’ activity of the nerves and cells
The researchers found that fine-tuning their motion to make them more agile results in greater therapeutic efficacy in paralyzed mice. The treatment also performed better during tests with human cells, indicating increased bioactivity and cellular signaling.
“Given that cells themselves and their receptors are in constant motion, you can imagine that molecules moving more rapidly would encounter these receptors more often,” Stupp continues. “If the molecules are sluggish and not as ‘social,’ they may never come into contact with the cells.”
Once the formula connects to the receptors, they trigger two cascading signals, both of which are critical to spinal cord repair. One boosts the axons, which scientists liken to electrical cables — increasing communication between the body and brain.
The second saves neurons by causing the proliferation of cells and promoting the regrowth of lost blood vessels and tissue. The therapy also induces myelin to rebuild and reduces scarring that prevents healing.
Scientists believe this injection could help prevent paralysis after road crashes, falls, sports accidents, and gunshot wounds. Moreover, Prof. Stupp believes the underlying discovery — that “supramolecular motion” is a key factor in bioactivity — may make the shot applicable to other causes of paralysis.
“The central nervous system tissues we have successfully regenerated in the injured spinal cord are similar to those in the brain affected by stroke and neurodegenerative diseases, such as ALS, Parkinson’s disease and Alzheimer’s disease,” the researcher concludes. “Beyond that, our fundamental discovery about controlling the motion of molecular assemblies to enhance cell signaling could be applied universally across biomedical targets.”

South West News Service writer Mark Waghorn contributed to this report.
Please would you update me on the progress made in terms of human trials. I have a quadraplegic brother who was injured approximately 4 years ago. Is he eligible for trials? He lives in South Africa.
Regards
Kate
Very newsworthy article but you have to click through to get any details, and it could use a little simplification for general audiences!