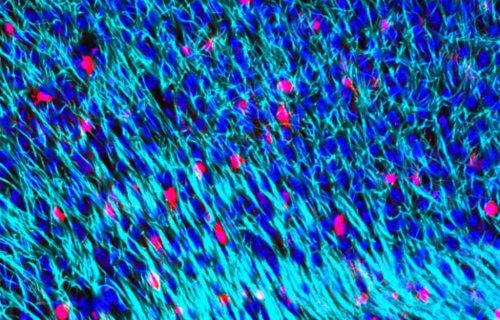
PORTSMOUTH, England — The depletion of a substance in the brain called myelin plays a key role in age-associated brain disorders, according to recent research. Myelin makes up the sheaths or coverings of the axons in the brain which are responsible for sending electrical signals. This covering allows signals to be sent from nerve cell to nerve cell as quickly as possible.
Researchers from the University of Portsmouth in England say that aging causes the cells responsible for repairing myelin to become inefficient. They also found the specific gene that codes for myelin replacement is negatively affected with age. The depletion of myelin leads to deterioration of the brain which causes neurodegeneration, responsible for diseases such as Parkinson’s disease and Alzheimer’s dementia.
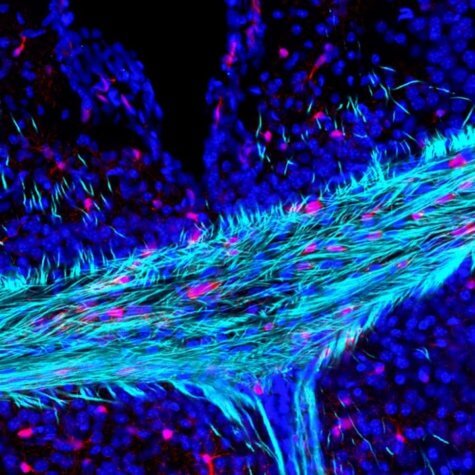
“Everyone is familiar with the brain’s gray matter, but very few know about the white matter, which comprises of the insulated electrical wires that connect all the different parts of our brains,” says study co-author Arthur Butt, a professor of cellular neurophysiology at Portsmouth, in a statement.
“A key feature of the aging brain is the progressive loss of white matter and myelin, but the reasons behind these processes are largely unknown,” he continues. “The brain cells that produce myelin – called oligodendrocytes – need to be replaced throughout life by stem cells called oligodendrocyte precursors. If this fails, then there is a loss of myelin and white matter, resulting in devastating effects on brain function and cognitive decline. An exciting new finding of our study is that we have uncovered one of the reasons that this process is slowed down in the aging brain.”
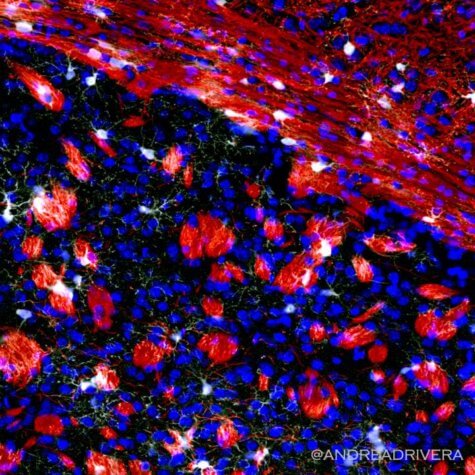
Dr. Andrea Rivera, who performed the key experiments for the study, says researchers examined the genome a young mouse brain and compared to that of an aging mouse.
“This very sophisticated analysis allowed us to unravel the reasons why the replenishment of oligodendrocytes and the myelin they produce is reduced in the aging brain,” says Rivera, who is now a Fellow at the University of Padua. “We identified GPR17, the gene associated with these specific precursors, as the most affected gene in the aging brain and that the loss of GPR17 is associated with a reduced ability of these precursors to actively work to replace the lost myelin.”
The study opens the door for future research into the possible regeneration of cells responsible for replenishing white matter. The team plans to focus on oligodendrocyte precursors in the next study, with hopes to develop a method by which these cells can be ‘rejuvenated’.
“This approach is promising for targeting myelin loss in the aging brain and demyelination diseases, including Multiple Sclerosis, Alzheimer’s disease, and neuropsychiatric disorders. Indeed, we have only touched the tip of the iceberg and future investigation from our research groups aim to bring our findings into human translational settings,” explains Dr. Kasum Azim of the University of Dusseldorf in Germany.
“MS can be relentless and painful, and there are sadly still no treatments to stop disability progression. We can see a future where no one has to worry about MS getting worse but, for that to happen, we need to find ways to repair damaged myelin. This research sheds light on why cells that drive myelin repair become less efficient as we age, and we’re really proud to have helped fund it,” adds Dr. Emma Gray, the assistant director of research at the MS Society. “By improving our understanding of aging brain stem cells, it gives us a new target to help slow the progression of MS, and could have important implications for future treatment,”
This study is published in the journal Ageing Cell.