BERLIN, Germany — The biological race between sperm to reach an egg is a fierce and competitive process. Now, researchers in Germany say they have discovered which protein gives sperm the winning edge. Their study finds a molecular “switch” in sperm could also allow men to turn their fertility on and off.
Experiments on mice find the “winner” carries a set of toxic mutations that poison rival sperm. Researchers say a genetic factor called “t-haplotype” promotes the success of the sperm carrying it. They are also fueled by a protein called RAC1, the molecular switch that propels sperm forward.
Could the sperm ‘switch’ boost or suppress fertility?
Sperm with this protein move faster than their peers, establishing an advantage in who reaches the egg first. The findings are expected to apply to humans as well. It could lead to a pill that boosts fertility in men, or a male oral contraceptive.
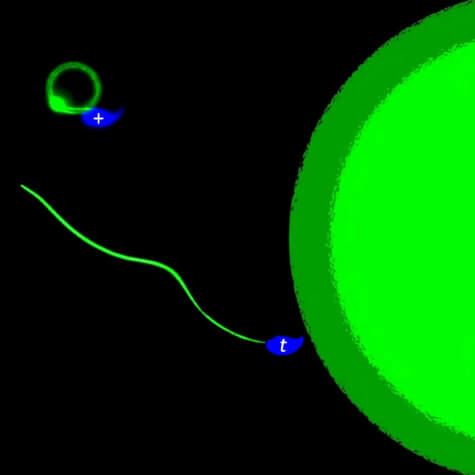
It would target this chemical, boosting or lowering levels. However, too much may cause male infertility. On average a man produces between 80 and 300 million sperm each time he ejaculates. Despite that, more than 60 percent of fertility issues are related to poor sperm, so it’s important to keep them healthy.
“Sperm with the t-haplotype manage to disable sperm without it,” says corresponding author Bernhard Herrmann of the Max Planck Institute for Molecular Genetics in a university release.
“The trick is that the t‑haplotype ‘poisons’ all sperm, but at the same time produces an antidote, which acts only in t-sperm and protects them. Imagine a marathon, in which all participants get poisoned drinking water, but some runners also take an antidote.”
Scientists find the ‘antidote’ for poor performing sperm
The study finds some of the genes carry mutations that distort regulatory signals, which then get distributed to all the sperm. These are the “poison” that disturbs progressive movement. The “antidote” comes into action after the set of chromosomes are split evenly between sperm during maturation, with each cell now containing only half.
Only those with the t-haplotype produce an additional factor that reverses the negative effect. Optimal amounts of RAC1 improve the competitiveness of individual sperm, offering fresh hope of combating male infertility.
It is literally a race for life when millions of sperm swim towards egg cells to fertilize them. Herrmann and colleagues described t-haplotype as a “selfish” and naturally occurring segment of DNA. It breaks the standard rules of genetic inheritance and awards a success rate of up to 99 percent to sperm cells containing it.
Analysis of individual sperm revealed most made only little progress on their paths and were genetically normal. On the other hand, straight moving sperm contained the t-haplotype. Crucially, RAC1 was identified as the key to differences in motility. It transmits signals from outside the cell to the inside by activating other proteins. RAC1 also directs sperm cells towards the egg, “sniffing” their way to the target.
“The competitiveness of individual sperm seems to depend on an optimal level of active RAC1; both reduced or excessive RAC1 activity interferes with effective forward movement,” first author Alexandra Amaral adds.
‘Ruthless competitors’
When the researchers treated the mixed population of sperm with a substance that inhibits RAC1, genetically “normal” sperm could now swim progressively. The advantage of t-sperm disappeared. The results explain why mice with two copies of the t-haplotype, one on each of the two chromosomes 17, are sterile.
They produce only sperm that carry the t-haplotype. These cells have much higher levels of active RAC1 than sperm from genetically normal mice. However, sperm from normal mice treated with the RAC1 inhibitor also lost their ability to move progressively. In other words, too little RAC1 activity is also disadvantageous. The researchers believe abnormal RAC1 activity might also be the underlying problem in forms of human infertility among men.
“Our data highlight the fact that sperm cells are ruthless competitors,” Herrmann says.
Furthermore, the example of the t-haplotype demonstrates how some genes use somewhat dirty tricks to get passed on.
“Genetic differences can give individual sperm an advantage in the race for life, thus promoting the transmission of particular gene variants to the next generation.”
The groundbreaking study appears in the journal PLoS Genetics.
SWNS writer Mark Waghorn contributed to this report.