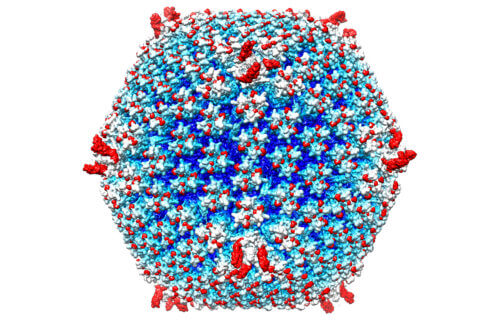
ATLANTA, Ga. — A “stealth bomber” virus could be the new killer weapon against cancer. While that may sound complicated, researchers from Emory University School of Medicine say the unique treatment is put together like a child’s toy.
Many cancer researchers have already devised “smart bombs” that allow a controlled release of two different anti-cancer drugs. Until now however, scientists say what’s been missing is the ability for these drugs to slip through the body’s defenses.
Now, researchers believe that the newly engineered virus could stamp out tumor growth in metastatic cancer patients.
“This is a new avenue for treatment of metastatic cancers,” says lead author Professor Dmitry Shayakhmetov in a media release. “You can arm it with genes and proteins that stimulate immunity to cancer, and you can assemble the capsid, a shell of the virus, like you’re putting in Lego blocks.”
A surprising obstacle in treating cancer
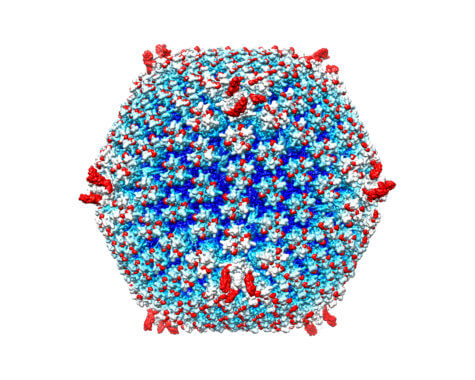
Cancer-killing viruses, or oncolytic viruses, have been studied and tested for decades. One such virus against melanoma was approved by the Food and Drug Administration in 2015. Scientists have always faced an overwhelming barrier in the fight against metastatic cancers however, that being the human immune system.
The body’s defense system quickly captures viruses injected into the blood stream and sends them to the liver, the body’s disposal unit. In this study, researchers reveal they can avoid these barriers by re-engineering the virus delivery system so the virus is not easily caught by the immune system. This makes it possible to inject the virus into the blood without causing a massive inflammatory reaction.
“The innate immune system is quite efficient at sending viruses to the liver when they are delivered intravenously,” Prof Shayakhmetov says. “For this reason, most oncolytic viruses are delivered directly into the tumor, without affecting metastases. In contrast, we think it will be possible to deliver our modified virus systemically at doses high enough to suppress tumor growth — without triggering life-threatening systemic toxicities.”
The study includes a structure of the re-engineered delivery system and shows the virus’s ability to eliminate tumors in mice. Scientists focused on re-engineering adenoviruses, a delivery system that has been used in dozens of cancer clinical trials to stimulate host anti-tumor response. Adenoviruses have also been central to gene therapy studies.
Inspired to make a better adenovirus
Prof. Shayakhmetov recalls the death of Jesse Gelsinger during a gene therapy clinical trial in 1999. The volunteer died of cytokine storm and multi-organ failure connected with high doses of an adenovirus vector delivered into the bloodstream.
Shayakhmetov adds this event inspired him to retool adenoviruses so they would not set off a strong inflammatory reaction. The study author views the re-engineered adenovirus as a platform technology, which can be adapted and customized for many types of cancer. It may even help individual cancer patients as a form of personalized cancer therapy.
Prof. Shayakhmetov started working on the modified virus technology at the University of Washington and founded a company, AdCure Bio, to bring a potentially life-saving therapy to patients with metastatic disease. In 2012, he published research on how adenovirus interacts with one host factor in the blood, coagulation factor X.
“Sometimes even small changes in structural proteins can be catastrophic and prevent assembly of the infectious virus,” adds co-study author Professor Phoebe Stewart of Case Western Reserve University.
“In this case, we modified adenovirus in three places to minimize virus interactions with specific blood factors. We found that the virus still assembles and remains functional for infecting and killing tumor cells.”
Promising results for killing tumors
It is still possible for a slower-building adaptive immune response to develop to the modified virus, similar to that observed with a vaccine. The researchers say a panel of viruses could be used among cancer patients to extend therapeutic benefits.
“Our study is the first to show that we can modify the binding of natural IgM to adenovirus,” Shayakhmetov explains. “We introduced mutations that prevent virus inactivation in the bloodstream and its trapping in liver macrophages, the largest pool of immune cells in our body that trap and destroy pathogens. Up to now, the prevailing view has been that any regular repeating structure, like the shell of the virus, would attract low-affinity natural IgM antibody binding, leading to its prompt inactivation and removal from the blood.”
The researchers also replaced part of the adenovirus that interacts with human cellular integrins. They substituted a sequence from another human protein that targets the virus-to-tumor cells. When injected into mice, high doses of standard adenovirus triggered liver damage and death within a few days, but the modified virus did not.
The modified virus could eliminate tumors from some, but not all mice engrafted with human lung cancer cells. A complete response – lack of detectable tumors and longer survival rate – occurred in about 35 percent of animals.
Scientists also discovered tumor sites in the lung transformed into scar tissue. Now, Prof. Shayakhmetov’s lab is exploring approaches to further increase the proportion of complete responders. He adds metastatic lung cancer would be the type of cancer most appropriate to test an oncolytic virus against.
The findings appear in the journal Science Translational Medicine.
SWNS writer Laura Sharman contributed to this report.