ST. LOUIS, Mo. — Could an implant help diabetic patients never have to worry about their blood sugar again? Researchers from Washington University School of Medicine in St. Louis and Cornell University have successfully subdued diabetes in a group of mice by implanting a tiny device capable of releasing insulin-secreting cells. Those imported cells only release insulin in response to blood sugar changes, meaning diabetes can be “reversed” without the need for any immune system-suppressing drugs.
“We can take a person’s skin or fat cells, make them into stem cells and then grow those stem cells into insulin-secreting cells,” says associate professor of medicine and co-senior investigator Jeffrey R. Millman, PhD, in a university release. “The problem is that in people with Type 1 diabetes, the immune system attacks those insulin-secreting cells and destroys them. To deliver those cells as a therapy, we need devices to house cells that secrete insulin in response to blood sugar, while also protecting those cells from the immune response.”
A cure for diabetes isn’t actually a new invention
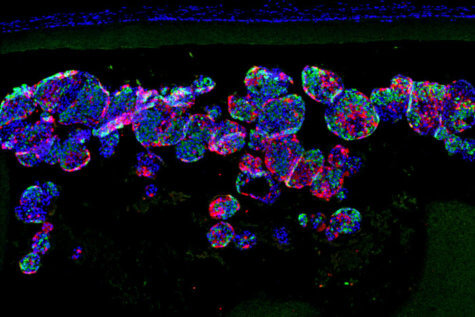
In previous work, Dr. Millman developed and perfected a way of creating induced pluripotent stem cells. From there, scientists are able to grow them into insulin-secreting beta cells. Those beta cells have successfully reversed diabetes in mice before, but researchers were uncertain how to safely recreate that process in human diabetes patients.
“The device, which is about the width of a few strands of hair, is micro-porous — with openings too small for other cells to squeeze into — so the insulin-secreting cells consequently can’t be destroyed by immune cells, which are larger than the openings,” Millman explains. “One of challenges in this scenario is to protect the cells inside of the implant without starving them. They still need nutrients and oxygen from the blood to stay alive. With this device, we seem to have made something in what you might call a Goldilocks zone, where the cells could feel just right inside the device and remain healthy and functional, releasing insulin in response to blood sugar levels.”
Scientists finally perfect the delivery system
For support, Millman and his team turned to Minglin Ma, PhD, an associate professor of biomedical engineering at Cornell. Dr. Ma and his team have been working on ways to safely implant beta cells into animals – with the hope that one day the same can be achieved for humans. Several implant devices have been tested in recent years, to varying degrees of success. This time around, researchers developed a unique “nanofiber-integrated cell encapsulation (NICE) device.” Study authors filled implants with insulin-secreting beta cells created via stem cells. The team then placed those devices into the abdomens of diabetic mice.
“The combined structural, mechanical and chemical properties of the device we used kept other cells in the mice from completely isolating the implant and, essentially, choking it off and making it ineffective,” Ma notes. “The implants floated freely inside the animals, and when we removed them after about six months, the insulin-secreting cells inside the implants still were functioning. And importantly, it is a very robust and safe device.”
The cells produced by the implants secreted insulin and controlled blood sugar in the lab mice for up 200 days. Notably, the cells continued functioning even though the mice hadn’t been given anything to suppress their immune systems.
“We’d rather not have to suppress someone’s immune system with drugs, because that would make the patient vulnerable to infections,” Millman concludes. “The device we used in these experiments protected the implanted cells from the mice’s immune systems, and we believe similar devices could work the same way in people with insulin-dependent diabetes.”
The study appears in the journal Science Translational Medicine.
